The Electron Arrangement of Any Particular Atom Shows
Question 15 The electron arrangement of any particular atom shows O the number of electrons in each energy level. For example fluorine has 9 electrons.

Chapter 2 Atomic Structure 2 1 Bohrs Atomic
The number of isotopes possible.

. 2 in the first electron shell and 7 in the second electron shell. The periodic table can be used to write the electron configuration for any element. O the number of isotopes possible.
The electron arrangement of any particular atom shows _____. E The maximum number of electrons each energy level can hold. Thus the electronic configuration of Chlorine Ne 3s2 3p5.
Chemistry questions and answers. For example the atomic number of sodium is 11. A Hydrogen atom has 1 proton and 1.
D a diagram of an atomic nucleus. A diagram of an atomic nucleus. The electron arrangement of any particular atom shows A the number of isotopes possible.
4 DOTS IN THE ELECTRON DOT STRUCTURE OF CARBON. What does the electron arrangement of any particular atom show. The arrangement of electrons in an atom determines its chemical properties and thus tells you what element it is.
The arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. Cl What is the electron energy level arrangement for Aluminum. The convention is to indicate the number of bonding electron pairs by the capital letter X the number of lone electron pairs by the capital letter E and the capital letter A for the central atom of the molecule AX n E mWhen predicting molecular geometry keep in mind the.
Lithium Li has an atomic number of 3 meaning that in a neutral atom the number of electrons will be 3. Sodium atoms have 11 protons and so 11 electrons. The number of electrons in each energy level Maximum number of electrons that may occupy the third electron energy level is 18 What is the element with the electron arrangement 287.
Therefore carbon is written as 2 4. The number of electrons in each energy level. ELECTRON ARRANGEMENT OF ANY ATOM SHOWS THE NUMBER OF ELECTRONS IN EACH ENERGY LEVEL.
The maximum number of electrons each energy level can hold. The number of the principal quantum shell n The letter that designates the orbital type the subshell l and. The trick is locate the particular element in the correct level and sublevel.
38 What is the element with the electron configuration A Be. The energy levels are shown as concentric circles around the central nucleus and the electrons are placed from the inside out. For the alkaline earth elements it is ns 2.
Hence Chlorine and Bromine have the same chemical and physical properties because both of them have seven electrons in their outermost shell. The electron level arrangement of an atom gives the number of electrons in each energy level. The atomic number of Bromine 35.
A A diagram of an atomic nucleus. For example carbon has 6 electrons 2 of these electrons will occupy the first energy level and the other 4 will occupy the second level. ATOMS ARE NEITHER CREATED NOR DESTROYED DURING A CHEMICAL.
We describe an electron configuration with a symbol that contains three pieces of information. E the maximum number of electrons each enerw level can hold. The number of the principal quantum shell n The letter that designates the orbital type the subshell l and.
Electron domain is used in VSEPR theory to determine the molecular geometry of a molecule. The electron arrangement of any particular atom shows a description of the shape of each electron energy level. C the number of electrons in each energy level.
D a diagram of an atomic nucleus. The arrangement of electrons in a lithium atom. E the maximum number of electrons each energy level can hold.
Bohr Models are used to show electron configurations of an atom and Lewis Dot Structures are used to show the arrangement of valence electrons in an element. B a description of the shape of each enerW level. ELECTRON ARRANGEMENT OF ANY ATOM SHOWS THE NUMBER OF ELECTRONS IN EACH ENERGY LEVEL.
The electron arrangement of any particular atom shows the number of electrons in each energy level Which element shown below could have chemical properties similar to magnesium. B a description of the shape of each energy level. A description of the shape of each electron energy level.
The electron arrangement of any particular atom shows _____. The arrangement of electrons in the first twenty elements can be written using the rules above and commas to separate each energy level. We say the general electron configuration for the alkali metals is ns 1.
The level numbers are located to the left of each period. We describe an electron configuration with a symbol that contains three pieces of information Figure 152. Thus the electronic configuration of Bromine Ar 3d104s24p5.
37 The electron arrangement of any particular atom shows A the number of isotopes possible. The arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. B The number of electrons in each energy level.
D The number of isotopes possible. The electron arrangement of an atom can be worked out from its atomic number. C A description of the shape of each electron energy level.
C the number of electrons in each energy level. Determine the number of electrons that the atom hasFill the s orbital in the first energy level the 1s orbital with the first two electronsFill the s orbital in the second energy level the 2s orbital with the second two electronsPut one electron in each of the three p orbitals in the second energy level the 2p orbitals and then if there are still electrons remaining go back. The maximum number of electrons each energy level can hold.

Chemistry 11 Electronic Structure Of The Atom

File Electron Shell 118 Ununoctium Svg Electron Configuration Atom Diagram Element Symbols

Atomic Model Task Cards Task Cards Electron Configuration Chemistry Activities

Electron Configuration Wikiwand

Some Fundamentals Of Mineralogy And Geochemistry Chemistry Redox Reactions Physics And Mathematics

See The Electron Configuration Diagrams For Atoms Of The Elements Electron Configuration Atom Diagram Element Symbols

The Electronic Structure Of The Atom Youtube

File Electron Shell 094 Plutonium Svg Wikimedia Commons Electron Configuration Atom Diagram Element Symbols

Electronegativity A Measure Of The Ability Of An Atom That Is Bonded To Another Atom To Attract Electrons To Itself Trend Increas Chemistry Atom Presentation

Chemical Bonding Atomic Structure And Bonding Britannica
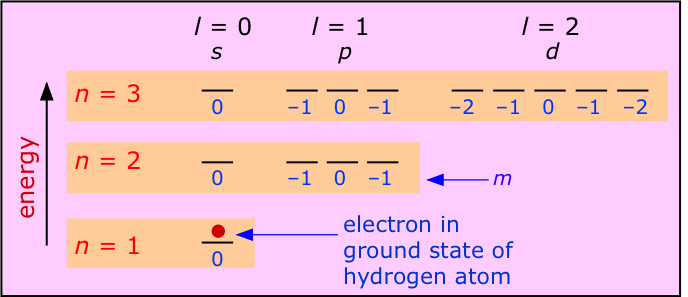
2 7 Atomic Electron Configurations Chemistry Libretexts

Ionization Energy Ionization Energy Electron Configuration Chemistry

Periodic Table Elements Element Facts Have Your Students Create A Storyboard With Information About An Element Atom Diagram Student Created Teacher Guides

Atom Structure Worksheet Middle School Google Search Teaching Chemistry Chemistry Classroom Chemistry Lessons

File Electron Shell 118 Ununoctium Svg Electron Configuration Atom Diagram Element Symbols

Electron Configurations Ppt Video Online Download

Top 100 Cool Science Facts For Kids Cool Science Facts Facts For Kids Interesting Science Facts

